Heat transfer by conduction
Mechanism
At all temperatures, heat energy (associated with temperature)
is transferred by conduction between any two points at
different temperatures when these points are connected by a heat
transfer medium. The medium may be a solid, a liquid, a gas, or
a plasma, i.e., the primary states of matter. Since macroscopic
temperature is a reflection of the thermal energy of the atoms
involved in the solid, the transfer medium simply provides a way
that atoms/molecules tend to share their thermal energy with those
around them; as a consequence, hotter regions become cooler because
they dissipate some of their thermal energy to surrounding cooler
regions which are in thermal contact.
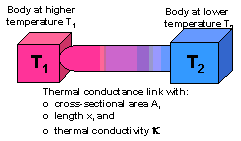
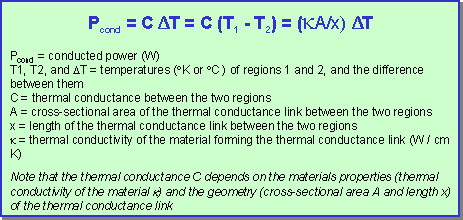
The conductive heat transfer between two regions or bodies at different temperatures is proportional to the temperature difference between the regions, with the proportionality constant dependent on the physical and dimensional properties of the material between them:


Dynamics
The time-dependent behavior of the system above is determined
by the fact that energy transfer (power) from the hotter to the
colder body is proportional to the temperature difference between
them at that time. Initially, then, the power transfer has its
highest value, but as the two bodies approach the same temperature
the rate of change is slower. This leads to an exponential approach
to the same final temperature for the two bodies. In addition,
the final temperature will depend not only on the initial temperatures
of the two bodies (and in reality of the thermal link between
them), but also on the masses of the bodies at the different temperatures:
if the cold body is much larger than the hot one, the hot one
will cool almost to that of the cold one, while if the masses
are the same the final temperature will be midway between the
two initial temperatures.
Generalization
This behavior actually appears much more broadly in engineering.
For a description, click
here.
Material properties
dependence
Conductive heat transfer may occur through solid materials, or
through liquids or gases or plasmas. In any case these materials
must be in contact with the bodies for conductive heat transfer
to take place between them. The thermal conductivity of gases
or plasmas is much less than that for solids or liquids, because
the separation between atoms or molecules in the gas phase is
far greater than in the solid or liquid phase. For example, the
thermal conductivity of aluminum is about 10,000 times larger
than that of air.
In addition, the thermal conductivity
of solids varies widely, dependent on the structure of the solid.
For example, metals such as Al and Cu have high values (2.37 and
4.01 W/cm-K), while insulators can have much lower values (quartz
[SiO2] at 0.0138) or relatively high values (Al2O3 at 0.363).
The thermal conductivity of gases depends significantly on their
pressure, because this determines their interatomic or intermolecular
spacing.